Introduction
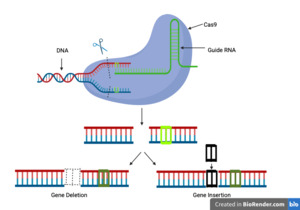
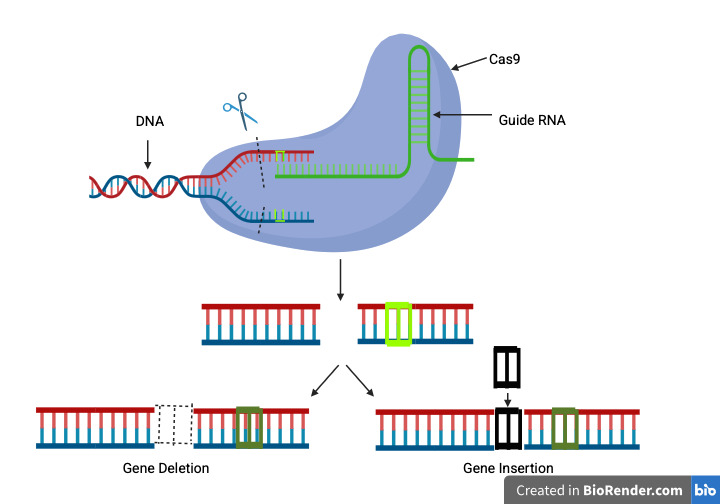
This research perspectives paper will analyze the challenges related to CRISPR gene therapy. CRISPR/Cas9 is a targeted genome editing technology that uses the Cas protein from certain species of bacteria and archaea. This can be used as a genetic treatment to individually modify certain genes inside of an organism. CRISPR has many uses ranging from helping agriculture to treating medical conditions such as cancer, allergic reactions to neurological issues. The wide applicability of CRISPR make it one of the most popular and important genetic editing technologies in today’s research landscape. To understand CRISPR we have to understand its origins. Despite seeming highly futuristic, genome editing has been around since 1990 with the discovery of genome-editing meganucleases. Genome editing meganucleases worked by targeting specific double-stranded DNA sequences and splitting them apart for editing. There were also other genetic editors, including zinc finger nucleases (ZFNs) and transcription activator-like effector nucleases (TALENs). These three gene editing applications can act as molecular "scissors’’ and they work by getting rid of individual genes, replacing them with other genes, or modifying them (Li et al., 2021). CRISPR works by creating a double strand break in DNA, and as the cell tries to repair that break, oftentimes one or more nucleotides are either inserted or deleted. This in turn causes a frameshift or nonsense mutation that can change the amino acids that the gene encodes (Figure 1).
Further research into CRISPR has led to improved gene editing technologies. Base editors are also used to change specific bases. This is done by fusing a Cas9 nuclease with an engineered base converter enzyme. The three kinds of base editors included cytosine base editors (CBEs), adenine bases editors (ABEs), glycosylase base editors (GBEs), and C-to-G base editors (CGBSe). Another type includes prime editors which fuse reverse transcriptase (RT) with a catalytically impaired Cas9 endonuclease. Prime editors are a search-and-replace editing technology that directly inputs a new genome sequence. They are relatively new and still need to be researched and modified (Kantor et al., 2020).
By understanding how CRISPR and other gene editors work, we can observe what sets CRISPR apart. This includes CRISPR’s increased specificity for protein-based DNA binding compared to other editors, making it more effective. In addition, CRISPR is highly modifiable, making it effective for a wide array of diseases and also having the added advantage of direct genome modification in the embryo and the potential to introduce multiple mutations at a time. With all this in mind, we have to understand how CRISPR gets into genes in the first place and that is through delivery methods. There are various delivery methods for CRISPR and the way they work is vital to whether the gene editor works at all. If the delivery method fails then the gene editing will not even be possible. These components are all vital to the process of using CRISPR as a genome editor and the way they work together is the only reason this process is possible. After learning about what CRISPR is and how it works we have to investigate the purpose of this paper, the issues of CRISPR. CRISPR, like many scientific tools, is still in its early stages, and it may be many more years until it is ready for mass usage due to the many issues plague this valuable tool. These issues boil down to two main aspects: the scientific and ethical problems of using gene editors.
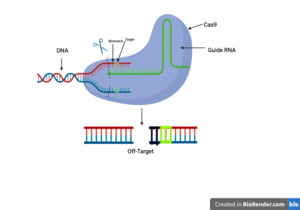
One important scientific problem of using gene editors is the danger of off-target effects. Off-target effects as its name suggests- are effects from using a gene editor on genes that weren’t supposed to be targeted. These issues mainly affect genes with similar sequences to the targeted gene, which in some cases can be accidentally modified as well. The consequences depend on where the off-target cutting occurs, but could potentially be harmful. Due to the possibility of off-target effects with no way to definitively prevent them, CRISPR’s use in human clinical research has been heavily set back making it nearly impossible to continue progress until this issue has been addressed. On top of this major issue, there are potential problems related to the efficiency and delivery of the gene editors.
These scientific issues, while problematic, are not more so than the ethical concerns. These concerns stem from the fear of defying nature. By changing the genes of an organism -we are directly defying nature and changing an organism in a way that was not intended. This causes some to question if gene editing should even be allowed, and when the line should be drawn. Concerns also stem from fear of “Designer Babies” which refers to a more dystopian belief that stems from the continuous improvement of people using gene editing until they become something that is not even human anymore. While these concerns are entirely valid, they can be prevented with proper regulation-but now we must ask how do we regulate this practice? A line must be drawn between helping people, and enhancing them. However, finding this line is what plagues many researchers around the globe. These issues, dystopian or not, are important and if we as a species wish to continue down this path they must be addressed. With these concerns- scientific and ethical alike- we can begin to understand what exactly must be done to help make this truly wonderful science into a possibility for all to benefit from.
Technical Challenges and Complications of Gene Editing
CRISPR is currently one of the biggest developments in genetic editing, but with its progress there are also many issues. Just recently in 2023, we saw the emergence of CRISPR in the clinical setting for the first time in the use of Casgevy which was created to treat sickle cell disease (Henderson, 2019). This development shows the massive growth CRISPR has made over the years and how it will only continue to grow. But despite this promising news it is still important to consider that CRISPR is still very young in its clinical stages.
CRISPR has made great strides since its inception in 1987, but it still has a few major issues that must be addressed if the field will continue to be researched. Gene editing is a powerful tool in addressing many major diseases that kill millions yearly, but it can prove to be just as deadly if the proper precautions are not taken. There have been a few documented cases of deaths due to complications of gene therapy, but one of the most famous by far is the unfortunate death of Jesse Gelsinger. Gelsinger did not die from CRISPR but instead from another gene therapy. However, his death still had an important role in affecting how genome editing would be conducted in humans. Gelsinger suffered from a metabolic disorder known as ornithine transcarbamylase and he underwent experimental gene therapy aimed to fix his condition. However, he suffered a severe immune reaction to the virus used as a delivery method, killing him (Sibbald, 2001). His death, while tragic, served as an extremely important reminder to the scientific community about how dangerous things like CRISPR can be if the proper precautions are not taken. Examples such as these reinforce why some of the key issues of CRISPR that still need to be solved include off-target effects and delivery methods (G et al., 2021).
Off-target effects are some of the most dangerous issues surrounding CRISPR due to the wide scope of the damage they can cause. CRISPR works by targeting certain sequences of DNA and manually editing them to cure certain diseases, but off-target effects happen when other sequences are unintentionally changed causing major complications (Guo et al., 2023). These off-target effects can be very harmful to patients and in some cases even fatal (Figure 2).
While off-target effects do pose a major threat they can be minimized in today’s CRISPR research. One important tool many researchers are using to combat off-target effects is in silico tools. In silico tools are online software that use complex prediction algorithms to calculate where off-target effects are most likely to occur. In silico tools are effective in finding many potential off-target effect areas, but that alone isn’t enough to prevent off-target effects. Many different additional experimental strategies aim to reduce off-target effects such as improving Cas, gRNA, and delivery methods. Starting with Cas improvement, there are many strains of Cas so by further developing and analyzing them the number of off-target effects can be greatly limited. The various Cas strains can serve different purposes for different diseases ensuring greater on-target effects (Naeem et al., 2020). Another way to reduce off-target effects is through improving guide RNA or gRNA. gRNA works as a director for the Cas proteins telling it where to bind and cut. By increasing the number of gRNA that is bound to the target site and its efficiency, Cas proteins will be less likely to produce off-target effects (Naeem et al., 2020). The final piece that can help reduce off-target effects is improving CRISPR delivery methods.
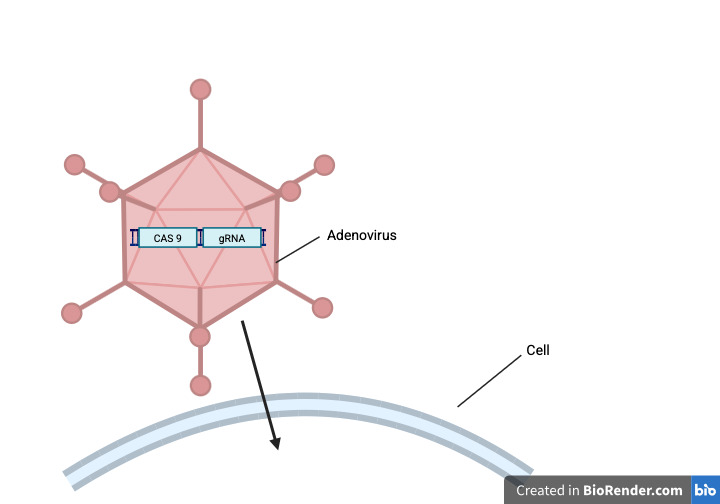
CRISPR cannot be injected directly into the nucleus by itself, but instead needs some kind of delivery method to carry it to its destination. These delivery methods are very important in ensuring that the Cas protein and gRNA get to where they need to go and do not produce the aforementioned off-target effects. Should the delivery service be ineffective or dangerous, it can prove fatal as seen by the death of Jesse Gelsinger. There are many different delivery methods associated with CRISPR, some viral and some non-viral. Viral delivery systems use a virus to transport DNA encoding gRNA and Cas9 into the target cells. This is effective in transporting the genetic cargo, but the issue stems from the fact that viral vectors are more persistent in the cell allowing the CRISPR to stay longer than necessary producing off-target effects. There are a few solutions in the market currently like the use of adenoviruses which have little potential in being able to integrate into the target cell (Naeem et al., 2020) (Figure 3). Another delivery alternative is non-viral delivery, which uses other methods to transport CRISPR but can still sometimes lead to overexpression of the genome editor. There are some solutions like using an RNP complex which delivers the Cas9 and gRNA as a pre-assembled compound, limiting the editing window preventing off-target effects (Naeem et al., 2020). Delivery methods are integral in transporting CRISPR so their development is crucial in ensuring the safety of patients and furthering CRISPR as a field.
CRISPR is a budding field in today’s research and will only continue to grow as time progresses but for it to grow properly these issues must be addressed. By improving Cas proteins and gRNA, off-target effects can be reduced at the source ensuring greater safety. This can further be accomplished by improving delivery methods and cutting down the genome editing window to prevent cases like Gelsinger’s from happening in the future. CRISPR will undoubtedly save millions of lives in the future but only if its issues are addressed.
Ethical considerations of Gene Editing
Humanity has always been evolving from our early primitive origins to our current 21st century state but what if that process could be accelerated? Starting as nothing but primitive apes and turning into such a complex intertwined society -humanity has always shown their capacity for growth and learning. However, this growth of human technology hasn’t happened at a steady rate. In fact, 300 years ago technology would not have changed much in 50 years, but now we have gone from simple landlines to complex smartphones in an even shorter time. This rapid growth begs the question of when we will grow too much for our own good. We are already seeing this in the field of genome editing has grown significantly in a short time. 15 years ago-the possibility of creating genetically modified children would not have been fathomable, but now that reality is within our reach. With this future that is fast approaching -we must stop and wonder if what we are doing is truly the right thing. There is no doubt in anyone’s mind that genome editing through CRISPR will become the next major stepping stone to make hereditary diseases and illnesses a thing of the past, but what if we go further than that? What if instead of merely erasing the issues humans face -we made them greater than they already are? If genome editing is left unchecked, it may result in a future where babies are created to be faster, stronger, and smarter than the previous generations until the very notion of a human will be obsolete. Before this research can go any further- humanity has to step in and think if what we are doing is ethically correct. There has to be a consensus reached on how to properly regulate this technology to allow humanity to thrive and prosper without our very own creations coming back to hurt us. With all these implications in mind, this section will demonstrate the various ethical perspectives on the topic of CRISPR genome editing as well as the existing and potential new regulations that need to be placed on this field of study.
Philosophically many problems arise from the use of CRISPR and other similar genome editors because it fundamentally violates what it means to be human. For thousands of years humans have evolved according to the laws of nature, changing based on fitness and selection, but now through the development of genome editors, there can be changes far beyond what is needed. This is where the philosophical argument comes in as it begs the question of whether CRISPR violates human dignity. Human dignity is a concept that states that all humans have an intrinsic value regardless of status, race, or wealth. Germline editing can violate human dignity as it gives certain people a measurable advantage over others from the moment they are born. Despite the possible violations of human dignity-the inherent medical benefits far outweigh the risks (Joseph et al., n.d.). Philosophically, germline editing can violate what it means to be human, but it is up to society to regulate and prevent that from happening.
Theologically, germline editing also brings up many questions related to the beliefs of people. There are hundreds of religions around the world but what many have in common is the belief in a higher power, a belief that humans and everything else in the universe were created for a purpose. With this in mind theologically, humans and all life are perfect in the eyes of religion as society was created based on the ideals of that higher power. This germline editing brings up many concerns among religious groups because interfering with the genome of living beings is directly changing God’s creation. There are many views on whether germline editing should be allowed from a religious perspective, but the common consensus among major religions is that germline editing should be used only for medical purposes and not for enhancement purposes (Joseph et al., n.d.). The religious works of most major religions support the fact that humans were created to live and thrive, which in turn means medicine and its many applications are allowed as they further life. Therapeutic germline editing is supported due to its promising potential to cure and prevent many diseases if safety and regulatory protocols are ensured. However, the issue again arises from the field of enhancement germline editing as that field is not necessary for the survival of humanity but only to better society’s goals. This field directly intervenes with God’s creations for no reason except human desire. Theologically, CRISPR editing can save many human lives, but decisions on what to use CRISPR editing for should be heavily weighed before going past just saving lives.
Finally, the arguably most important field regarding CRISPR ethics is social ethics. Social ethics is broader than philosophy or theology as it does not cover just one set of ideals, but instead the overarching view of the general public. Among the billions of people around the world, there are many differing viewpoints and beliefs on what to do about germline editing, especially due diverse education on the subject. When people think of things like CRISPR or gene editing, they may think of science-fiction or fantasy where there are super-beings due to having changed genes. While that may be possible in a couple hundred years, it is nowhere near the scope of what is currently being researched. Fortunately, knowledge of genome editing and CRISPR has become more mainstream in recent years, allowing for more focused discussion regarding this topic. A recurring theme among many groups of people is their overall support of therapeutic germline editing, but an innate fear of hereditary genome editing and its effects on future generations (Joseph et al., n.d.). Many believe in the goal of saving lives through genome editing, but are opposed to the amount of damage future generations may suffer due to the actions done currently.
With all the various ethical perspectives around this field, the recurring theme among all of them is their belief that human lives should be saved but not at the risk of unregulated, dangerous practices. That is why proper regulations are needed for CRISPR and other similar genome editors as they continue developing. Issues arise in the determination of how strict these regulations should be. Science flourishes from the creativity of people and their ideas so overly strict regulations would prevent the field’s proper growth. On the other hand, mismanaged research could easily turn into dangerous eugenics research. Regulations surrounding CRISPR are currently managed by prominent scientific bodies in collaboration with regulatory agencies like the FDA. Currently, risks of CRISPR clinical research turning into eugenics are managed by the scientific community which is prioritizing life-threatening diseases (Gabel & Moreno, 2019). This will only last for a short time though and eventually, greater regulations will need to be implemented to allow for the safe continuation of CRISPR research.
Overall, the ethical consensus surrounding CRISPR is the strong belief in the good it can cause in its medical applications as well as a growing sense of fear of what could happen should things go wrong. Most major branches of ethics have come to a consensus regarding the fact that CRISPR is needed to save human lives but it should not be blindly followed without proper safety and regulatory controls. Regulations surrounding germline editing and CRISPR are still relatively weak due to this being such a revolutionary field. These regulations likely will not be good enough in the coming years. CRISPR is a highly important field in the future of humanity but it should be discussed thoroughly to ensure it does not do more harm than good.
Discussion
CRISPR has made great progress since its discovery almost forty years ago but with that much progress, there are plenty of scientific and ethical issues. CRISPR is a very powerful tool but it can also be extremely dangerous if the proper precautions are not taken. CRISPR’s major scientific issues stem from its capability to cause off-target effects as well as improper delivery services causing problems. These issues can be fatal if left unchecked making them important to address. Fortunately, through continued research, these problems are being solved through new developments like in silico tools which can find off-target effects and improved viral delivery services that prevent continued gene editing. CRISPR is a powerful scientific tool that will improve through continued study. But despite these concerns, many people worry about the more ethical issues with human genome editing. There are many different ethical perspectives from theological to cultural, each focusing on their viewpoints on the research of genome editing. Despite these various perspectives the common consensus tends to be that genome editing is an extremely important tool in helping humanity throughout the future, but it should not be taken towards the greater depths of human enhancement. The major ethical argument stems from the belief that human life is precious and should be saved at any cost which supports the use of CRISPR and other genetic research, but this ethical argument also believes that human enhancement through CRISPR is something that shouldn’t be done as it violates what a human truly is. These ethical concerns stem from a common human fear for the future but by addressing and working with these fears in mind CRISPR and other editors will continue to become more widely accepted. CRISPR will undoubtedly save lives in the future but humanity shouldn’t be swayed by its potential and ignore the threats it can pose. By exposing and eliminating this threat CRISPR and genome editing as a whole will become a scientific field that helps humanity to a large degree.